|
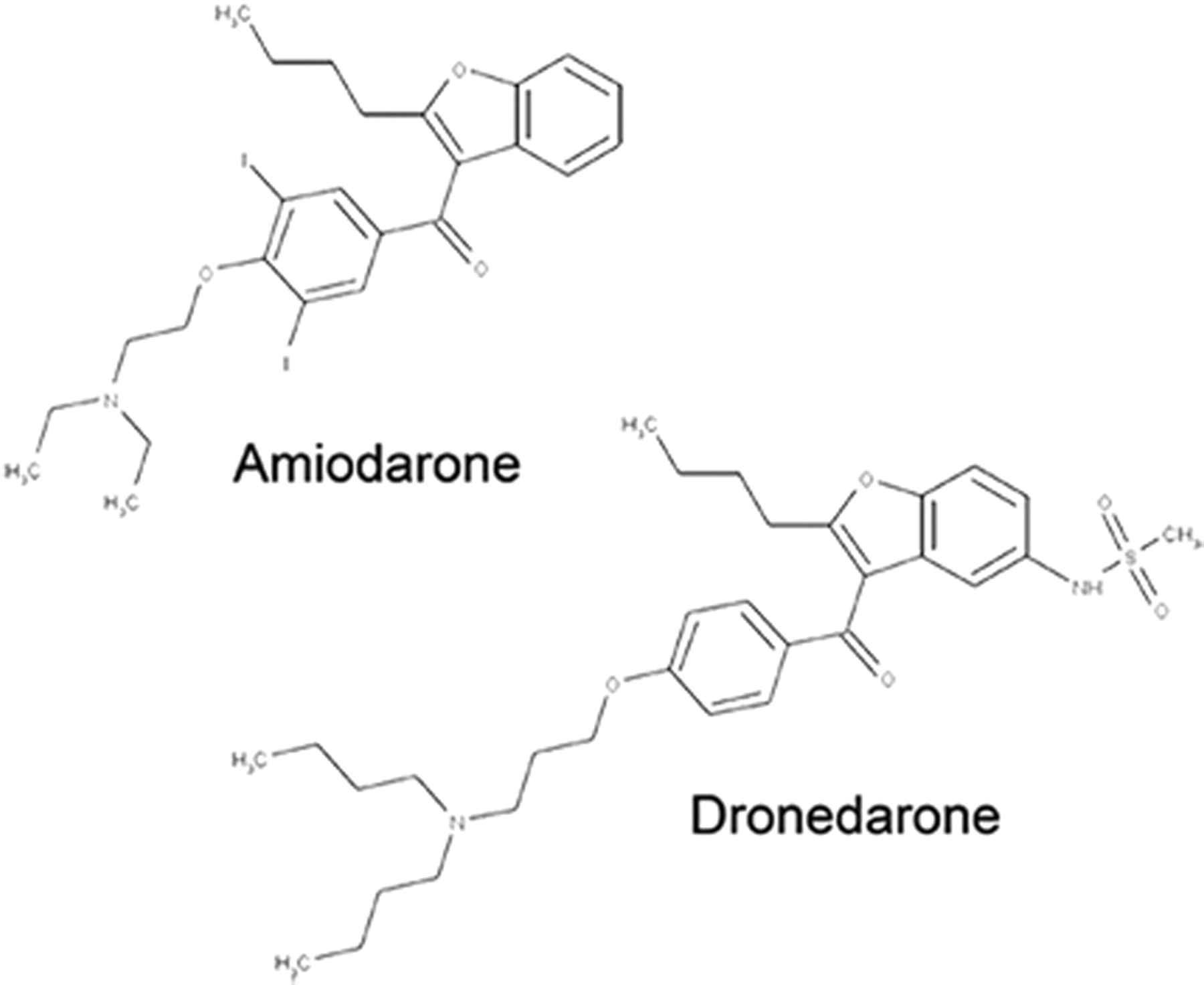
Order Tramadol Online Overnight Amiodarone |
|
| MOA: Class III; http://economiacircularverde.com/que-es-la-economia-circular/ non-iodinated antiarrhythmic agent structurally related to amiodarone. Inhibits sodium and potassium channels. | https://www.petwantsclt.com/petwants-charlotte-ingredients/ MOA: Class III, Purchase Tramadol Overnight iodinated & inhibits adrenergic stimulation, affects sodium and potassium channels |
| Beers Criteria:
use caution or avoid in older adults |
Beers Criteria:
use caution or avoid in older adults |
| Indications:
Atrial fibrillation (paroxysmal or persistent) |
Indications:
Atrial fibrillation, ventricular arrhythmias (pulseless & stable), Pre & post prophylaxis for operation on heart, cardiopulmonary resuscitation |
| Warnings:
– Heart failure – Severe liver injury – Qtc prolongation – BBW: permanent Afib – Renal effects – Respiratory effects (pulmonary fibrosis) |
Warnings:
– Life threatening cutaneous reactions – BBW: hepatotoxicity – Ocular effects – Photosensitivity – BBW: Pro-arrhythmic effects – BBW: pulmonary toxicity – Thyroid effects |
| Dose = 400 mg PO BID | Depends on indication; e.g. Ventricular tachycardia maintenance dose is 100-200mg daily |
| Pregnancy:
Contraindicated in pregnancy |
Pregnancy:
Can use in pregnancy if benefit outweighs risk |
| Hepatic impairment:
No dosage adjustment mild to moderate, contraindicated in severe Renal impairment: No dosage adjustment |
Hepatic Impairment:
Dosage adjustment is probably necessary but no guidelines available Renal Impairment: No dosage adjustment |
| Discontinue strong CYP3A4 inhibitors |